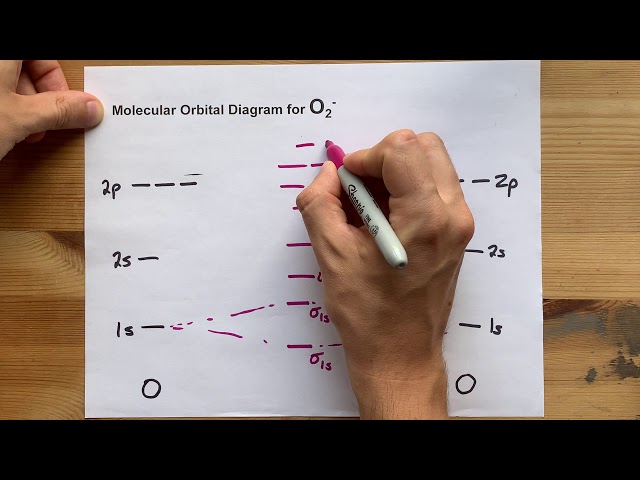
O2 Molecular Orbital Diagram
An O2 molecular orbital diagram is a visual representation of the molecular orbitals of an oxygen molecule. It shows the energy levels, shapes, and symmetries of the orbitals and how electrons are distributed among them. Molecular orbital theory is a quantum mechanical model that describes the electronic structure of molecules. It provides a framework for understanding chemical bonding, molecular geometry, and many other properties of molecules.
To create an O2 molecular orbital diagram, the following steps can be followed:
- Write out the electron configuration of each atom in the molecule.
- Combine the atomic orbitals of the atoms to form molecular orbitals.
- Determine the energy of each molecular orbital.
- Determine the symmetry of each molecular orbital.
- Fill the molecular orbitals with electrons.
The resulting molecular orbital diagram will show the energy levels, shapes, and symmetries of the molecular orbitals, as well as how the electrons are distributed among them.
Benefits of using an O2 molecular orbital diagram
- Provides a visual representation of the molecular orbitals of a molecule.
- Shows the energy levels, shapes, and symmetries of the orbitals.
- Helps to understand chemical bonding and molecular geometry.
- Can be used to predict the properties of molecules.
Tips for creating an O2 molecular orbital diagram
- Start by understanding the basics of molecular orbital theory.
- Use a periodic table to determine the electron configuration of each atom in the molecule.
- Use orbital hybridization to combine the atomic orbitals of the atoms.
- Use molecular orbital theory to determine the energy and symmetry of each molecular orbital.
- Fill the molecular orbitals with electrons according to the Aufbau principle.
By following these steps, you can create an O2 molecular orbital diagram that will help you to understand the electronic structure of an oxygen molecule.
O2 Molecular Orbital Diagram
An O2 molecular orbital diagram is a visual representation of the molecular orbitals of an oxygen molecule. It shows the energy levels, shapes, and symmetries of the orbitals and how electrons are distributed among them. Molecular orbital theory is a quantum mechanical model that describes the electronic structure of molecules. It provides a framework for understanding chemical bonding, molecular geometry, and many other properties of molecules.
- Energy levels: The molecular orbital diagram shows the energy levels of the molecular orbitals. The energy levels are determined by the interaction between the atomic orbitals that combine to form the molecular orbitals.
- Shapes: The molecular orbital diagram shows the shapes of the molecular orbitals. The shapes of the molecular orbitals are determined by the symmetry of the atomic orbitals that combine to form the molecular orbitals.
- Symmetries: The molecular orbital diagram shows the symmetries of the molecular orbitals. The symmetries of the molecular orbitals are determined by the symmetry of the atomic orbitals that combine to form the molecular orbitals.
- Electron distribution: The molecular orbital diagram shows how electrons are distributed among the molecular orbitals. The electron distribution is determined by theaufbau principle.
- Bonding: The molecular orbital diagram can be used to understand chemical bonding. The bonding orbitals are the molecular orbitals that are filled with electrons. The number of bonding electrons determines the bond order of the molecule.
- Antibonding: The molecular orbital diagram can be used to understand antibonding. The antibonding orbitals are the molecular orbitals that are not filled with electrons. The number of antibonding electrons determines the antibond order of the molecule.
- Molecular properties: The molecular orbital diagram can be used to predict the properties of molecules. The properties of a molecule are determined by the electronic structure of the molecule.
The seven key aspects of an O2 molecular orbital diagram are essential for understanding the electronic structure of an oxygen molecule. These aspects can be used to understand chemical bonding, molecular geometry, and many other properties of molecules.
Energy levels
The energy levels of the molecular orbitals are important because they determine the chemical properties of the molecule. For example, the energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) determines the molecule’s reactivity. The HOMO is the orbital that is most likely to donate electrons, while the LUMO is the orbital that is most likely to accept electrons. The smaller the energy difference between the HOMO and LUMO, the more reactive the molecule.
The energy levels of the molecular orbitals can also be used to understand the molecular geometry of the molecule. For example, the VSEPR theory predicts that the molecular geometry of a molecule is determined by the number of electron pairs in the valence shell of the central atom. The electron pairs in the valence shell will occupy the lowest energy molecular orbitals, and the molecular geometry will be the one that minimizes the electron-electron repulsion.
The energy levels of the molecular orbitals are a fundamental property of molecules. They determine the chemical properties, molecular geometry, and many other properties of molecules. The molecular orbital diagram is a powerful tool for understanding the electronic structure of molecules.
Shapes
The shapes of the molecular orbitals are important because they determine the overlap between the orbitals and the strength of the chemical bond. The overlap between two orbitals is greatest when the orbitals have the same shape and are aligned with each other. The greater the overlap, the stronger the chemical bond.
- Sigma bonds are formed when the orbitals overlap head-to-head. Sigma bonds are the strongest type of chemical bond.
- Pi bonds are formed when the orbitals overlap sideways. Pi bonds are weaker than sigma bonds.
- Delta bonds are formed when the orbitals overlap sideways and have a nodal plane between them. Delta bonds are the weakest type of chemical bond.
The molecular orbital diagram can be used to predict the shapes of the molecular orbitals and the types of chemical bonds that will be formed. For example, the O2 molecular orbital diagram shows that the two oxygen atoms are bonded by two sigma bonds and one pi bond. This is because the two oxygen atoms have two unpaired electrons in their 2p orbitals. The two 2p orbitals overlap head-to-head to form a sigma bond, and the two 2p orbitals overlap sideways to form a pi bond.
The shapes of the molecular orbitals are a fundamental property of molecules. They determine the types of chemical bonds that will be formed and the strength of those bonds. The molecular orbital diagram is a powerful tool for understanding the electronic structure of molecules.
Symmetries
The symmetry of a molecular orbital refers to its behavior under certain symmetry operations, such as rotations, reflections, and inversions. The symmetry of a molecular orbital is important because it can be used to determine the properties of the molecule, such as its polarity, magnetic susceptibility, and spectroscopic properties.
- Sigma orbitals are symmetric with respect to rotation about the internuclear axis. They are also symmetric with respect to reflection through a plane containing the internuclear axis.
- Pi orbitals are antisymmetric with respect to rotation about the internuclear axis. They are also antisymmetric with respect to reflection through a plane containing the internuclear axis.
- Delta orbitals are antisymmetric with respect to both rotation about the internuclear axis and reflection through a plane containing the internuclear axis.
The O2 molecular orbital diagram shows that the two oxygen atoms are bonded by two sigma bonds and one pi bond. This is because the two oxygen atoms have two unpaired electrons in their 2p orbitals. The two 2p orbitals overlap head-to-head to form a sigma bond, and the two 2p orbitals overlap sideways to form a pi bond. The pi bond is antisymmetric with respect to reflection through a plane containing the internuclear axis. This means that the pi bond has a nodal plane, which is a plane where the electron density is zero.
The symmetry of the molecular orbitals can be used to understand the properties of the O2 molecule. For example, the fact that the pi bond is antisymmetric with respect to reflection through a plane containing the internuclear axis means that the O2 molecule is paramagnetic. This means that the O2 molecule has unpaired electrons, and it is attracted to a magnetic field.
Electron distribution
The Aufbau principle states that electrons fill the lowest energy orbitals first. This means that the electrons in an O2 molecule will fill the 1g orbital first, then the 1u* orbital, then the 2g orbital, and so on. The molecular orbital diagram shows how the electrons are distributed among the molecular orbitals, and this information can be used to understand the chemical properties of the molecule.
For example, the O2 molecule has two unpaired electrons in the 2g orbital. This makes the O2 molecule paramagnetic, meaning that it is attracted to a magnetic field. The paramagnetism of the O2 molecule is important for its biological function, as it allows the molecule to bind to hemoglobin in red blood cells.
The electron distribution in an O2 molecule can also be used to understand the molecule’s reactivity. For example, the O2 molecule is a strong oxidizing agent because it has two unpaired electrons. These unpaired electrons can react with other molecules to form free radicals, which can damage cells.
The electron distribution in an O2 molecule is a fundamental property of the molecule. This information can be used to understand the chemical properties, reactivity, and biological function of the molecule.
Bonding
The molecular orbital diagram is a powerful tool for understanding chemical bonding. It can be used to predict the bond order, bond length, and bond strength of a molecule. The bond order is a measure of the strength of the bond between two atoms. The bond length is the distance between the nuclei of two atoms. The bond strength is the energy required to break the bond between two atoms.
- Bond Order: The bond order of a molecule is determined by the number of bonding electrons. A bond order of 1 indicates a single bond, a bond order of 2 indicates a double bond, and a bond order of 3 indicates a triple bond. The O2 molecular orbital diagram shows that the two oxygen atoms are bonded by a double bond. This is because the two oxygen atoms have two bonding electrons.
- Bond Length: The bond length of a molecule is determined by the strength of the bond. A stronger bond will have a shorter bond length. The O2 molecular orbital diagram shows that the bond length of the O-O bond is 121 pm. This is because the O-O bond is a strong bond.
- Bond Strength: The bond strength of a molecule is determined by the energy required to break the bond. A stronger bond will have a higher bond strength. The O2 molecular orbital diagram shows that the bond strength of the O-O bond is 498 kJ/mol. This is because the O-O bond is a strong bond.
The molecular orbital diagram is a valuable tool for understanding chemical bonding. It can be used to predict the bond order, bond length, and bond strength of a molecule. This information can be used to design new molecules with specific properties.
Antibonding: The molecular orbital diagram can be used to understand antibonding. The antibonding orbitals are the molecular orbitals that are not filled with electrons. The number of antibonding electrons determines the antibond order of the molecule.
The molecular orbital diagram of O2 shows that there are two antibonding orbitals, 1u and 3g. These orbitals are not filled with electrons. The antibond order of O2 is 2, which means that the O-O bond is a double bond. The antibonding orbitals weaken the O-O bond, but they are not strong enough to break the bond.
The antibonding orbitals play an important role in the chemistry of O2. For example, the 1u orbital is involved in the reaction of O2 with hemoglobin. Hemoglobin is a protein that carries oxygen in the blood. The 1u orbital accepts electrons from hemoglobin, which allows O2 to bind to the protein. The 3g orbital is involved in the reaction of O2 with other molecules. For example, the 3g orbital accepts electrons from carbon monoxide, which forms a stable complex with O2. This complex prevents O2 from binding to hemoglobin, which can lead to carbon monoxide poisoning.
The molecular orbital diagram is a powerful tool for understanding the chemistry of molecules. It can be used to predict the properties of molecules, such as their bond strength and reactivity. The molecular orbital diagram can also be used to design new molecules with specific properties.
Molecular properties
The molecular orbital diagram of O2 can be used to predict a variety of molecular properties, including the bond length, bond strength, and magnetic susceptibility. The bond length is determined by the number of bonding electrons and the strength of the bond. The bond strength is determined by the energy difference between the bonding and antibonding orbitals. The magnetic susceptibility is determined by the number of unpaired electrons in the molecule.
- Bond length: The O2 molecular orbital diagram shows that the O-O bond length is 121 pm. This is because the O-O bond is a double bond, which means that there are two bonding electrons. The bonding electrons are in the 1g and 2g orbitals.
- Bond strength: The O2 molecular orbital diagram shows that the O-O bond strength is 498 kJ/mol. This is because the O-O bond is a double bond, which means that there is a large energy difference between the bonding and antibonding orbitals.
- Magnetic susceptibility: The O2 molecular orbital diagram shows that the O2 molecule has two unpaired electrons in the 2g orbital. This means that the O2 molecule is paramagnetic, which means that it is attracted to a magnetic field.
The molecular orbital diagram is a powerful tool for understanding the properties of molecules. It can be used to predict a variety of molecular properties, including the bond length, bond strength, and magnetic susceptibility. This information can be used to design new molecules with specific properties.
An O2 molecular orbital diagram is a visual representation of the molecular orbitals of an oxygen molecule. It shows the energy levels, shapes, and symmetries of the orbitals and how electrons are distributed among them.
Molecular orbital diagrams are important because they provide a deeper understanding of the electronic structure and bonding of molecules. They can be used to predict a variety of molecular properties, such as bond length, bond strength, and magnetic susceptibility. Molecular orbital diagrams are also used in the design of new molecules with specific properties.
In the case of the O2 molecule, the molecular orbital diagram shows that the two oxygen atoms are bonded by a double bond. This is because the two oxygen atoms have four valence electrons, which fill the two bonding orbitals and the two non-bonding orbitals. The molecular orbital diagram also shows that the O2 molecule is paramagnetic, which means that it is attracted to a magnetic field.
FAQs on O2 Molecular Orbital Diagram
Here are some frequently asked questions about O2 molecular orbital diagrams:
Question 1: What is an O2 molecular orbital diagram?
An O2 molecular orbital diagram is a visual representation of the molecular orbitals of an oxygen molecule. It shows the energy levels, shapes, and symmetries of the orbitals and how electrons are distributed among them.
Question 2: Why are molecular orbital diagrams important?
Molecular orbital diagrams are important because they provide a deeper understanding of the electronic structure and bonding of molecules. They can be used to predict a variety of molecular properties, such as bond length, bond strength, and magnetic susceptibility. Molecular orbital diagrams are also used in the design of new molecules with specific properties.
Question 3: What does the O2 molecular orbital diagram tell us about the bonding in the O2 molecule?
The O2 molecular orbital diagram shows that the two oxygen atoms are bonded by a double bond. This is because the two oxygen atoms have four valence electrons, which fill the two bonding orbitals and the two non-bonding orbitals.
Question 4: Why is the O2 molecule paramagnetic?
The O2 molecular orbital diagram shows that the O2 molecule has two unpaired electrons in the 2g orbital. This means that the O2 molecule is paramagnetic, which means that it is attracted to a magnetic field.
Question 5: How can I draw an O2 molecular orbital diagram?
To draw an O2 molecular orbital diagram, you need to know the electron configuration of the oxygen atom. The electron configuration of oxygen is 1s2 2s2 2p4. The 2p orbitals are the valence orbitals, and they will interact to form the molecular orbitals of the O2 molecule.
Question 6: What are some applications of molecular orbital diagrams?
Molecular orbital diagrams are used in a variety of applications, including the design of new molecules with specific properties, the study of chemical reactions, and the understanding of the electronic structure of materials.
Summary: O2 molecular orbital diagrams are a powerful tool for understanding the electronic structure and bonding of molecules. They can be used to predict a variety of molecular properties and are used in the design of new molecules with specific properties.
Transition to the next article section: For more information on molecular orbital diagrams, please see the following resources:
- Wikipedia: Molecular orbital diagram
- ChemPRIME: Molecular Orbital Diagrams
- Mastering Chemistry: Molecular Orbital Diagrams
Conclusion
The O2 molecular orbital diagram is a powerful tool for understanding the electronic structure and bonding of the oxygen molecule. It provides a visual representation of the molecular orbitals, their energy levels, shapes, and symmetries, and how electrons are distributed among them.
The O2 molecular orbital diagram can be used to predict a variety of molecular properties, such as bond length, bond strength, and magnetic susceptibility. It can also be used to understand the reactivity of the oxygen molecule and its role in biological processes.
The O2 molecular orbital diagram is a valuable tool for chemists and other scientists who are interested in understanding the electronic structure and bonding of molecules.
Youtube Video: