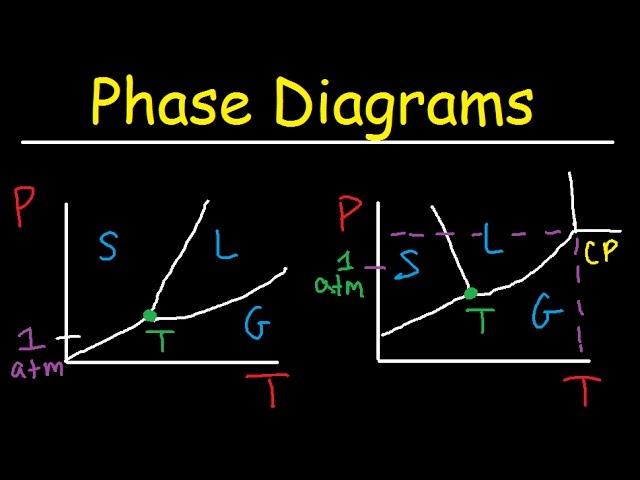
Phase Diagram of Water
A phase diagram is a graphical representation of the thermodynamic conditions at which different phases of a substance coexist in equilibrium. The phase diagram of water is a particularly important diagram because water is such a common substance and because it exhibits a number of unusual properties, such as its high specific heat capacity and its ability to exist in three different phases (solid, liquid, and gas) at room temperature and pressure.
The phase diagram of water can be used to predict the behavior of water under a variety of conditions. For example, it can be used to determine the melting point and boiling point of water, as well as the conditions under which water will freeze, melt, or vaporize. The phase diagram can also be used to design experiments and to interpret experimental data.
There are a number of different ways to create a phase diagram of water. One common method is to plot the pressure of water against its temperature. This type of diagram is called a P-T diagram. Another common method is to plot the temperature of water against its volume. This type of diagram is called a T-V diagram.
The following are some of the benefits of using a phase diagram of water:
- Can be used to predict the behavior of water under a variety of conditions
- Can be used to determine the melting point and boiling point of water
- Can be used to design experiments and to interpret experimental data
Tip 1: Choose the appropriate type of phase diagram for your needs. There are a number of different types of phase diagrams, each with its own advantages and disadvantages. The type of phase diagram that you choose will depend on the information that you are interested in obtaining. Tip 2: Gather the necessary data. In order to create a phase diagram, you will need to gather data on the pressure, temperature, and volume of water. This data can be obtained from a variety of sources, such as books, journals, and websites. Tip 3: Plot the data on a graph. Once you have gathered the data, you will need to plot it on a graph. The type of graph that you use will depend on the type of phase diagram that you are creating. Tip 4: Analyze the graph. Once you have plotted the data, you will need to analyze the graph to identify the different phases of water. The phases of water will be represented by different lines or curves on the graph. Tip 5: Draw the phase diagram. Once you have identified the different phases of water, you can draw the phase diagram. The phase diagram will show the conditions under which the different phases of water coexist in equilibrium.
Phase Diagram of Water
A phase diagram of water is a graphical representation of the thermodynamic conditions at which different phases of water coexist in equilibrium. It is a valuable tool for understanding the behavior of water under various conditions and has applications in many fields, including chemistry, physics, and engineering.
- Pressure-temperature relationship: The phase diagram shows the relationship between the pressure and temperature at which water exists in different phases.
- Triple point: The triple point is the point on the phase diagram where the solid, liquid, and gas phases of water coexist in equilibrium.
- Critical point: The critical point is the point on the phase diagram beyond which liquid and gas phases cannot be distinguished.
- Sublimation: Sublimation is the process by which water changes directly from a solid to a gas, bypassing the liquid phase.
- Melting: Melting is the process by which water changes from a solid to a liquid.
- Boiling: Boiling is the process by which water changes from a liquid to a gas.
- Condensation: Condensation is the process by which water changes from a gas to a liquid.
- Freezing: Freezing is the process by which water changes from a liquid to a solid.
These key aspects of the phase diagram of water provide a comprehensive understanding of the behavior of water under different conditions. They are essential for predicting the behavior of water in various applications, such as power plants, chemical processing, and refrigeration systems.
Pressure-temperature relationship
The pressure-temperature relationship is a fundamental aspect of the phase diagram of water. It shows how the pressure and temperature of water affect its phase behavior. This information is essential for understanding the behavior of water in various applications, such as power plants, chemical processing, and refrigeration systems.
- Vapor pressure: The vapor pressure of water is the pressure at which water vapor coexists in equilibrium with liquid water. The vapor pressure increases with increasing temperature.
- Boiling point: The boiling point of water is the temperature at which water vapor pressure equals the external pressure. At the boiling point, water boils and turns into steam.
- Melting point: The melting point of water is the temperature at which ice melts and turns into liquid water. The melting point decreases with increasing pressure.
- Triple point: The triple point is the point on the phase diagram where the solid, liquid, and gas phases of water coexist in equilibrium. The triple point of water is at a temperature of 0.01 C and a pressure of 0.006 atm.
These are just a few of the important insights that can be gained from the pressure-temperature relationship on the phase diagram of water. By understanding this relationship, we can better understand the behavior of water and predict its behavior under different conditions.
Triple point
The triple point is a unique and important point on the phase diagram of water. It is the only point at which all three phases of water can coexist in equilibrium. The triple point of water is at a temperature of 0.01 degrees Celsius and a pressure of 0.006 atmospheres. These conditions are very close to the conditions found on the surface of the Earth, which is why water is so common in all three phases on our planet.
-
Facet 1: The triple point and the phase diagram
The triple point is a critical point on the phase diagram of water. It is the point at which the solid, liquid, and gas phases of water are all in equilibrium. This means that the three phases can coexist without any net change in the system. The triple point is also the point at which the three phase boundaries (solid-liquid, liquid-gas, and solid-gas) meet.
-
Facet 2: The triple point and water’s properties
The triple point of water is a very important point because it determines many of water’s properties. For example, the triple point determines the melting point and boiling point of water. The triple point also determines the density of water in its solid, liquid, and gas phases.
-
Facet 3: The triple point and water’s behavior
The triple point of water is also important because it affects the behavior of water in many different situations. For example, the triple point determines the way that water freezes and melts. The triple point also determines the way that water evaporates and condenses.
The triple point of water is a very important point on the phase diagram of water. It is a critical point that determines many of water’s properties and behavior. By understanding the triple point, we can better understand water and its role in our world.
Critical point
The critical point is a unique and important point on the phase diagram of water. It is the point at which the liquid and gas phases of water become indistinguishable from each other. This means that at the critical point, water has properties that are intermediate between those of a liquid and a gas. For example, at the critical point, water has a density that is equal to the density of its vapor, and its refractive index is equal to the refractive index of its vapor.
The critical point of water is located at a temperature of 374.15 degrees Celsius and a pressure of 22.064 megapascals. These conditions are far beyond the conditions that are normally found on the surface of the Earth. However, the critical point of water is important for understanding the behavior of water in many different situations, such as in power plants and chemical processing plants.
The critical point of water is also important for understanding the behavior of water in the Earth’s atmosphere. At high altitudes, the temperature and pressure of the atmosphere can be very close to the critical point of water. This can lead to the formation of clouds and precipitation.
Understanding the critical point of water is important for many different reasons. It is important for understanding the behavior of water in a variety of applications, and it is also important for understanding the behavior of water in the Earth’s atmosphere.
Sublimation
Sublimation is a unique and important process in the phase diagram of water. It is the process by which water changes directly from a solid to a gas, bypassing the liquid phase. This process is important for understanding the behavior of water in a variety of applications, such as freeze-drying and snowmaking.
-
Facet 1: Sublimation and the phase diagram
Sublimation is represented by a line on the phase diagram of water. This line separates the solid and gas phases of water. The slope of this line indicates the rate at which sublimation occurs. The steeper the slope, the more rapidly sublimation occurs.
-
Facet 2: Sublimation and water’s properties
Sublimation affects many of water’s properties. For example, sublimation can cause water to lose mass. Sublimation can also cause water to change its density and volume.
-
Facet 3: Sublimation and water’s behavior
Sublimation affects the behavior of water in a variety of ways. For example, sublimation can cause water to freeze and melt. Sublimation can also cause water to evaporate and condense.
Sublimation is a complex and fascinating process. By understanding sublimation, we can better understand the behavior of water and its role in our world.
Melting
Melting is a fundamental process in the phase diagram of water. It is the process by which water changes from a solid to a liquid. This process is important for understanding the behavior of water in a variety of applications, such as heating and cooling systems, and water treatment plants.
-
Facet 1: Melting and the phase diagram
Melting is represented by a line on the phase diagram of water. This line separates the solid and liquid phases of water. The slope of this line indicates the rate at which melting occurs. The steeper the slope, the more rapidly melting occurs.
-
Facet 2: Melting and water’s properties
Melting affects many of water’s properties. For example, melting can cause water to lose mass. Melting can also cause water to change its density and volume.
-
Facet 3: Melting and water’s behavior
Melting affects the behavior of water in a variety of ways. For example, melting can cause water to freeze and melt. Melting can also cause water to evaporate and condense.
Melting is a complex and fascinating process. By understanding melting, we can better understand the behavior of water and its role in our world.
Boiling
Boiling is a fundamental process in the phase diagram of water. It is the process by which water changes from a liquid to a gas. This process is important for understanding the behavior of water in a variety of applications, such as power plants, chemical processing plants, and water treatment plants.
-
Facet 1: Boiling and the phase diagram
Boiling is represented by a line on the phase diagram of water. This line separates the liquid and gas phases of water. The slope of this line indicates the rate at which boiling occurs. The steeper the slope, the more rapidly boiling occurs.
-
Facet 2: Boiling and water’s properties
Boiling affects many of water’s properties. For example, boiling can cause water to lose mass. Boiling can also cause water to change its density and volume.
-
Facet 3: Boiling and water’s behavior
Boiling affects the behavior of water in a variety of ways. For example, boiling can cause water to freeze and melt. Boiling can also cause water to evaporate and condense.
-
Facet 4: Boiling and the environment
Boiling plays an important role in the environment. For example, boiling is the process by which water evaporates from the oceans and lakes. This water vapor then condenses to form clouds and rain.
Boiling is a complex and fascinating process. By understanding boiling, we can better understand the behavior of water and its role in our world.
Condensation
Condensation is an important process in the water cycle. It is the process by which water vapor in the air turns into liquid water. Condensation occurs when the air is cooled to the point where it can no longer hold all of the water vapor. The water vapor then condenses into liquid water droplets.
-
Condensation and the phase diagram of water
Condensation is represented by a line on the phase diagram of water. This line separates the gas and liquid phases of water. The slope of this line indicates the rate at which condensation occurs. The steeper the slope, the more rapidly condensation occurs.
-
Condensation and water’s properties
Condensation affects many of water’s properties. For example, condensation can cause water to lose mass. Condensation can also cause water to change its density and volume.
-
Condensation and water’s behavior
Condensation affects the behavior of water in a variety of ways. For example, condensation can cause water to freeze and melt. Condensation can also cause water to evaporate and condense.
-
Condensation and the environment
Condensation plays an important role in the environment. For example, condensation is the process by which water vapor evaporates from the oceans and lakes. This water vapor then condenses to form clouds and rain.
Condensation is a complex and fascinating process. By understanding condensation, we can better understand the behavior of water and its role in our world.
Freezing
Freezing is an important process in the phase diagram of water. It is the process by which water changes from a liquid to a solid. This process is important for understanding the behavior of water in a variety of applications, such as refrigeration, air conditioning, and cryogenics.
On the phase diagram of water, freezing is represented by a line that separates the liquid and solid phases of water. The slope of this line indicates the rate at which freezing occurs. The steeper the slope, the more rapidly freezing occurs.
Freezing affects many of water’s properties. For example, freezing can cause water to lose mass. Freezing can also cause water to change its density and volume.
Freezing plays an important role in the environment. For example, freezing is the process by which water freezes on the surface of lakes and ponds. This ice can insulate the water below, preventing it from freezing solid. Freezing also plays a role in the formation of glaciers and ice caps.
Understanding freezing is important for many reasons. It is important for understanding the behavior of water in a variety of applications, and it is also important for understanding the behavior of water in the environment.
A phase diagram of water is a graphical representation of the thermodynamic conditions at which different phases of water coexist in equilibrium. It is a powerful tool for understanding the behavior of water under varying conditions, and it has applications in many fields, including chemistry, physics, and engineering.
One of the most important aspects of a phase diagram of water is that it can be used to predict the behavior of water under different conditions. For example, it can be used to determine the melting point and boiling point of water, as well as the conditions under which water will freeze, melt, or vaporize. This information is essential for designing and operating a wide range of systems and processes that involve water.
In addition to its predictive capabilities, a phase diagram of water can also be used to understand the historical context of water’s behavior. For example, it can be used to explain why ice floats on water, and why water expands when it freezes. This information can help us to better understand the behavior of water in natural systems, such as lakes and oceans.
FAQs about Phase Diagram of Water
A phase diagram of water is a graphical representation of the thermodynamic conditions at which different phases of water coexist in equilibrium. It is a valuable tool for understanding the behavior of water under various conditions and has applications in many fields, including chemistry, physics, and engineering.
Q
A: A phase diagram of water can provide information about the melting point, boiling point, and freezing point of water. It can also be used to determine the conditions under which water will exist as a solid, liquid, or gas.
Q
A: A phase diagram of water can be used to predict the behavior of water under different conditions. For example, it can be used to determine the temperature at which water will boil or freeze at a given pressure.
Q
A: A phase diagram of water has applications in many fields, including chemistry, physics, and engineering. It can be used to design and operate systems and processes that involve water, such as power plants, chemical processing plants, and refrigeration systems.
Q
A: A phase diagram of water is a simplified representation of the behavior of water. It does not take into account all of the factors that can affect the behavior of water, such as impurities and dissolved gases.
Q
A: One common misconception is that a phase diagram of water is only useful for predicting the behavior of pure water. In reality, a phase diagram of water can also be used to predict the behavior of water that contains impurities.
Summary
A phase diagram of water is a valuable tool for understanding the behavior of water under various conditions. It can be used to predict the behavior of water, design and operate systems and processes that involve water, and understand the historical context of water’s
Next Section
Conclusion
Conclusion
The phase diagram of water is a powerful tool that can be used to understand the behavior of water under different conditions. It is a valuable resource for scientists, engineers, and anyone else who works with water. By understanding the phase diagram of water, we can better predict the behavior of water in different systems and processes.
In this article, we have explored the phase diagram of water in detail. We have discussed the different phases of water, the conditions under which each phase exists, and the applications of the phase diagram of water. We have also discussed some of the limitations of the phase diagram of water and some of the most common misconceptions about it.
We hope that this article has helped you to better understand the phase diagram of water. We encourage you to continue learning about this important topic. The phase diagram of water is a fascinating and complex subject that can teach us a great deal about the behavior of water. By understanding the phase diagram of water, we can better understand the world around us.
Youtube Video: